Like in the nitrocefin assay, bacteria from routine agar cultures are investigated. CarbaLux Xtract buffer I is added to the tubes containing already the fluorescent substrate followed by suspending a full loop of bacteria in each tube. After few minutes at room temperature up to one hour of incubation at 37 °C, the result is already visible under a laboratory UV lamp. When present, the extracted bacterial resistance enzymes (in this case carbapenemases) rapidly degrade carbapenem CF with the decay of its fluorescence. Then the tubes appear dark gray in UV light. In the absence of carbapenemase, the test is negative and the samples remain yellow-green fluorescent (Fig. 1).
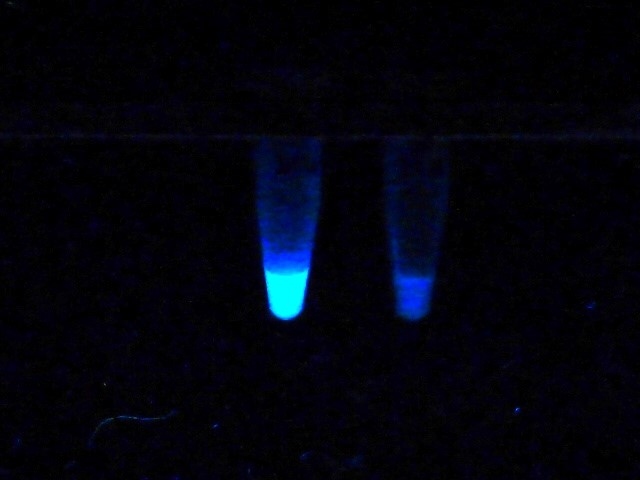
Fig. 1: Negative and positive test reaction in UV light.
An investigation with more than 200 asserted bacterial isolates and a practical evaluation of a microbiological laboratory from a hospital network revealed that the tests were highly functional and suitable.
The features of the CarbaLux Tests are:
- In difference to genotypic (PCR) tests there is no need to pre-select the target genes. Consequently, the phenotypic CarbaLux test can detect all carbapenemases (potentionally even to date unknown) - there are hundreds of variants - including unusual or less reactive ones.
- The fluorescence method allows the application of very low substrate concentrations. In consequence, the test becomes highly sensitive.
- OXA carbapenemases are very abundant in Europe and difficult to detect. This type of resistance is transferable to other bacteria. Because of the high sensitivity the test can provide proof of such variants.
- Many carbapenem hydrolyzing multiresistant bacteria, in particular Enterobacter spp., do not harbour classical carbapenemases but hyper-produced AmpC-betalactamases which were not covered by conventional rapid phenotypic or genotypic assays and thus distributed world-wide. With the introduction of the CarbaLux Test CF a reliable and simple method for their rapid detection becomes available. This type of resistance can be further identified by the AmpC supplemental Test Clox.
- The bacteria can be cultured over-night on different ready prepared agar plates. The additional expenditure of time is largely compensated by reliability, because viable and visible bacteria are investigated. Therefore, false negative results can be avoided.
An analytical device for the determination of the fluorescence intensity and the course of the fluorescence degradation is under development.
CarbaLux® is a registered trademark of CarbaLux GmbH
Imprint - Data Privacy Statement DSGVO
|

